Boyle’s Law—Inquiry Lab Kit For AP® Physics 2
$71.29 Original price was: $71.29.$49.90Current price is: $49.90.
- Experience the Best Quality
- Shop with Confidence, Pay Safely
- Shop quality, shop with us.
- The quality solution for all your needs.

Product Details
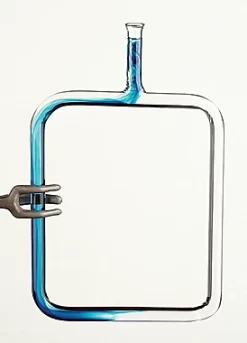
AP Physics 2, Big Idea 5, Investigation 3
Physics and chemistry often overlap, perhaps most significantly in their ability to form joint explanations of the behavior of gases. The four variables used to describe a gas—pressure, volume, temperature and moles—are intimately related. As one or more variables change, one or more of the others also change, in direct or inverse relation. This advanced-inquiry investigation explores the relationship between the pressure of a gas and the volume of the container in which it is held, as well as how a gas performs work or is worked upon.
The purpose of this advanced inquiry investigation is to derive a mathematical equation describing the relationship between the pressure and volume of a gas at constant temperature. In the introductory part of the experiment, students learn how to set up a special apparatus and quantify the amount of applied force to compress the gas. In the guided-inquiry portion of the experiment, students are challenged to derive the relationship between pressure and volume by constructing graphs, which they must further interpret to determine the amount of work done on or by the gas.
Complete for 24 students working in groups of three. All materials are reusable.
Be the first to review “Boyle’s Law—Inquiry Lab Kit For AP® Physics 2” Cancel reply
Related products
Heat & Thermodynamics
Physics & Physical Science
FlinnPREP™ Inquiry Lab Kits For AP® Physics 1: Measuring G, Exploring Freefall
Physics & Physical Science
Electricity
Forces & Equilibrium
Physics & Physical Science
Physics & Physical Science
Optics & Light














Reviews
There are no reviews yet.